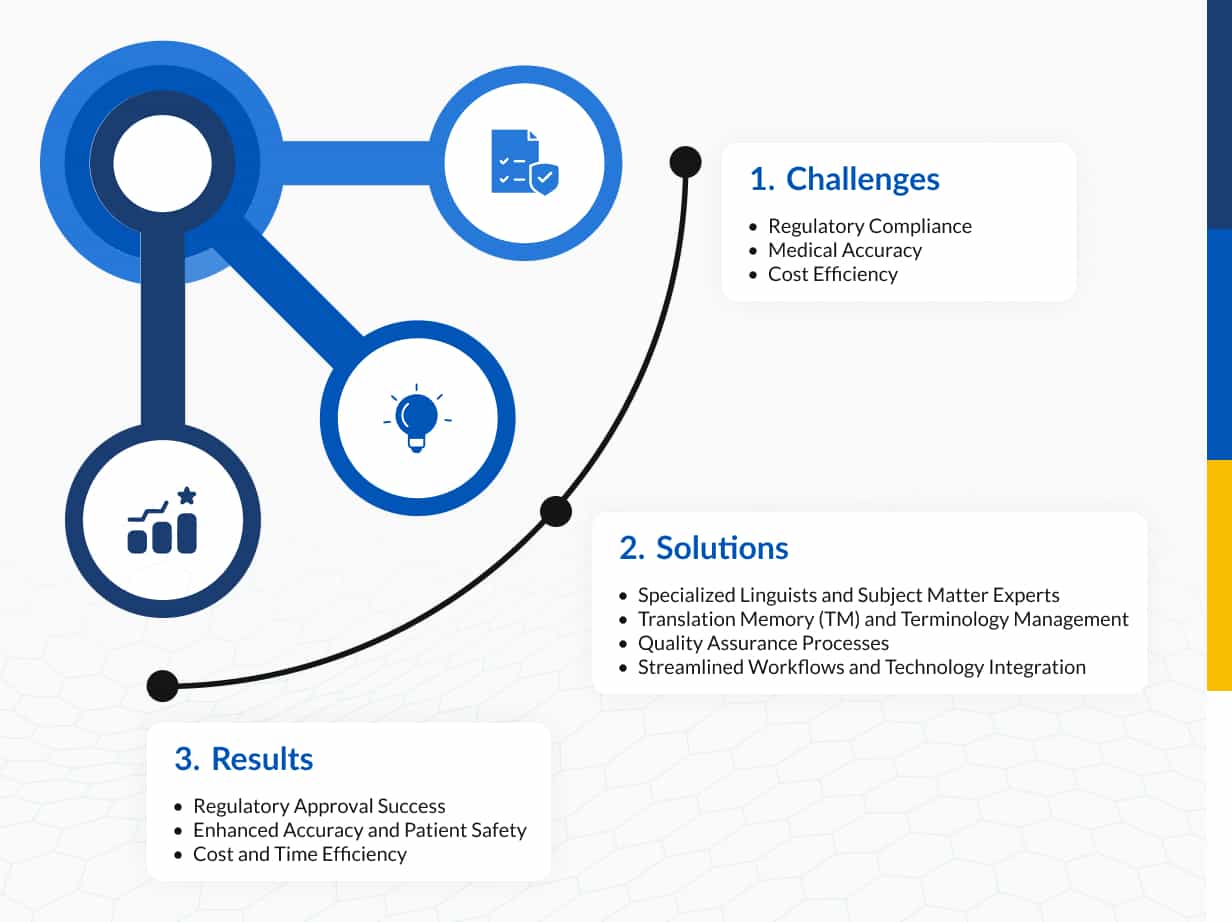
Challenges
Regulatory Compliance
The translation of clinical trials must adhere to stringent international and national regulations. Any deviation from these standards can result in delays, additional costs, or regulatory rejection.
Medical Accuracy
Clinical trial documentation includes highly technical content, such as protocols, informed consent forms, investigator brochures, and adverse event reports. A single mistranslation can lead to misinterpretation, compromising patient safety and trial integrity.
Cost Efficiency
Life sciences companies operate within tight budgets and timelines. High-quality translations must be delivered cost-effectively while maintaining consistency across multiple trial phases and languages.
Solutions
Specialized Linguists and Subject Matter Experts
Ciklopea ensures translation accuracy by employing linguists with specialized expertise in medicine, pharmaceuticals, and regulatory affairs. These professionals undergo rigorous vetting and continuous training to stay up to date with industry changes.
Translation Memory (TM) and Terminology Management
To enhance efficiency and consistency, Ciklopea leverages Translation Memory (TM) technology. TM stores previously translated content, reducing redundancy and ensuring uniform terminology across documents. This is particularly valuable when translating template-based documents, such as Informed Consent Forms (ICFs) or regulatory documentation, where large portions of text are repeated across versions. Additionally, Ciklopea develops client-specific glossaries and term bases to maintain consistency and compliance with regulatory terminology.
Quality Assurance Processes
Ciklopea’s multi-step quality assurance (QA) process includes:
- Bilingual Review: Ensuring alignment between source and target texts.
- Automated QA Tools: Identifying inconsistencies, errors, and formatting issues before final delivery.
Streamlined Workflows and Technology Integration
Ciklopea utilizes a centralized translation management system that automates project handling, assigns the best-suited linguists, and tracks progress in real-time. This system optimizes workflows, reducing turnaround times without compromising quality.
Results
Regulatory Approval Success
By ensuring compliance with regulatory standards, Ciklopea has helped life sciences companies avoid costly delays and secure approvals for their clinical trials in multiple markets.
Enhanced Accuracy and Patient Safety
The involvement of specialized linguists and robust QA processes has significantly minimized translation errors, ensuring that medical professionals and patients receive accurate and clear information.
Cost and Time Efficiency
Through the use of TM, terminology management, and automation, Ciklopea has reduced translation costs while accelerating delivery timelines. Clients have reported significant savings and improved efficiency in managing multilingual clinical trial documentation.
Conclusion
Translating clinical trial documentation requires a strategic approach that balances regulatory compliance, accuracy, and cost efficiency. Ciklopea’s expertise, technology-driven solutions, and commitment to quality have made it a trusted partner in the life sciences industry, ensuring successful trial execution across global markets.
If you are looking for a reliable translation partner for your clinical trial needs, Ciklopea is here to help. Contact us today to learn more about our life sciences translation services.